Golimumab (brand name Simponi) is an injectable biologic medication licensed to treat adults with moderate to severe ulcerative colitis. Find out more about it in this article.
Golimumab - also known by its brand name of Simponi - is an injectable biologic medication licensed to treat adults with moderate to severe ulcerative colitis who have failed to, or have not tolerated, other medications. It is made by Janssen Biotech. It is not currently licensed for use in Crohn's disease in the UK, however some doctors may prescribe it for Crohn's.
Crohn’s disease and ulcerative colitis have been linked to an increase in certain proteins - including one called tumor necrosis factor (TNF). Your body’s immune system naturally produces TNF.
An increase in TNF is linked to an increase in inflammation in the body’s digestive system - which can lead to a worsening of inflammatory bowel disease (IBD) symptoms.
Golimumab is one of a group of medications which target TNF proteins, bind to them and block them. This helps to prevent inflammation in the body which is hoped will reduce IBD symptoms. The immune system is also suppressed. This can mean that people taking golimumab are more prone to picking up illnesses and infections.
Simponi has been licensed for use in the UK in adults (over 18) who have moderate to severe ulcerative colitis and have not responded to other medications. The safety of Simponi use in under 18s hasn’t yet been established.
Before taking Simponi you should tell your doctor all of your symptoms and if you believe you may currently have any infections.
You should also tell your doctor if you are on any other medications, over-the-counter medicines, vitamins or supplements, including herbal supplements.
You should especially tell your doctor if you are taking:
If you are considered eligible to take Simponi your doctor will carry out some tests. This include tests for tuberculosis (TB) and hepatitis B. If these tests come back clear and your doctor has no other concerns then you may be eligible for Humira.
Simponi is taken via a subcutaneous injection every 4 weeks. Subcutaneous means that an injection is given into the fat between your skin and muscle (usually on your thigh or stomach).
Some people administer the injection themselves at home, while others receive it from a nurse. If you are considered suitable to self-administer you will receive full training from a qualified professional who will teach you how to do it and observe you the first couple of times.
You will receive three starter injections - two injections on the first day of treatment, followed by one injection two weeks later. After these you will be required to have one injection every four weeks.
If you are doing the injections yourself you will have them delivered to your home. They should be kept in their original packaging in the fridge between 2c and 8c. Before injecting you should remove the Simponi from the fridge and leave it for 30 minutes to in its packaging (to protect it from the light) so it can reach room temperature. If needed (such as if you are going on holiday) it can be stored at room temperature (up to 25c) for one period of time up to 30 days. It should not be put back into the fridge after this.
How long, and whether or not, a medication takes to works varies from person-to-person.
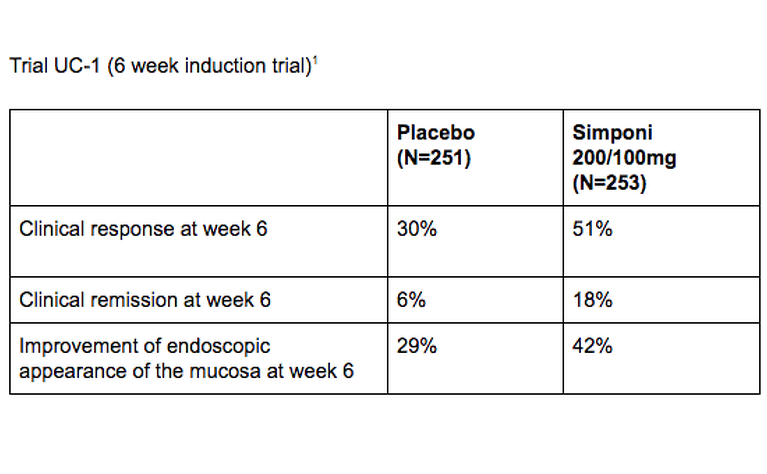
In a clinical trial half of patients receiving a 200mg dose followed by 100mg dose 2 weeks later were experiencing a clinical response to the drug at 6 weeks, with 18% in clinical remission.
Simponi is sometimes prescribed at the same time as other medications - such as corticosteroids or methotrexate, particularly if you have rheumatoid arthritis (RA) or psoriatic arthritis (PsA).
However, you should also tell your doctor if you are on any other medications, over-the-counter medicines, vitamins or supplements, including herbal supplements, before you start taking Simponi.
You should especially tell your doctor if you are taking:
People taking Simponi shouldn’t receive live vaccines or treatment with a weakened bacteria (such as BCG for bladder cancer).
Common side effects of Simponi include:
Other, more serious side effects, can include:
Children and adults taking TNF blockers - such as Simponi - there may be an increased risk in getting lymphoma or other cancers. You should tell your doctor if you have had, or develop, lymphoma or other cancers.
Some people taking Simponi have developed some types of skin cancer during or after treatment.
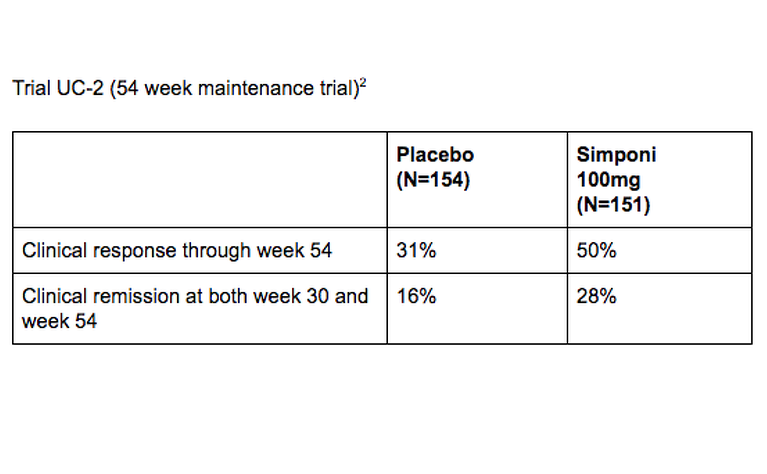
The safety and efficacy of Simponi were evaluated in two1,2 multi-centre, randomised, double-blind, placebo-controlled clinical trials in adults with ulcerative colitis. Below are the results from those trials.
1. Sandborn et al. Subcutaneous golimumab induces clinical response and remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014 Jan;146(1):85-95; quiz e14-5. doi: 10.1053/j.gastro.2013.05.048. Epub 2013 Jun 2. https://www.ncbi.nlm.nih.gov/pubmed/23735746
2. Sandborn et el. Subcutaneous golimumab maintains clinical response in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2014 Jan;146(1):96-109.e1. doi: 10.1053/j.gastro.2013.06.010. Epub 2013 Jun 14. https://www.ncbi.nlm.nih.gov/pubmed/23770005