Biosimilars have increased competition in the biologics market, making them more a more cost-effective treatment than previously. But, does this mean there will be more access to this treatment for patients? We take a look.
The introduction of biosimilars - medicines that are very similar to an original biologic therapy which has come off patent - has increased competition in the biologics market. An increase in competition has resulted in a decrease in the cost of this type of therapy, the most expensive in inflammatory bowel disease (IBD). So, does this mean more inflammatory bowel disease patients will be prescribed biologics at an earlier stage in their treatment? We take a look.
The introduction of biologics has changed treatment for many patients with conditions such as Crohn’s disease and ulcerative colitis. These more targeted treatments have provided another option for patients with moderate to severe disease who don’t respond to some of the other medications previously available in IBD. But biologic medicines are expensive, and this has been one of the main barriers to their more widespread use throughout the world. However, since the introduction of cheaper biosimilars 10 years ago things have started to slowly change, with the cost of this type of therapy reducing significantly.
In 2017/18 the NHS in England spent a total of £2.88 billion on the top 15 prescribed medicines, with biologics (and biosimilars) making up 83% of this spend1. During this same period the NHS saved £324m through switching to biosimilars or generic medications for 10 medicines (and almost £100m of this was through using more infliximab biosimilars)2. Based on this, it was estimated that by encouraging prescribing of the cheaper biologics and biosimilars the NHS in England could reduce this bill by at least £400-£500m annually by 2020-213.
This is no surprise. Biosimilars are priced 15-75% lower than the originator4. A small study in Finland5 found switching patients on infliximab to a biosimilar had a cost saving of two thirds.
Biosimilars occur when the original biologic medicine comes off patent. This means other companies can create biosimilars - versions of the drug that are very similar. In IBD, biosimilars infliximab and adalimumab have both come off patent. What was once a market monopolised by just a few pharmaceutical companies is now opening up.
In 2019 there were over 1,000 biosimilars in development6, meaning even more competition (and potential cost savings) might be on the horizon. When their products came off patent the makers of infliximab (Janssen) and adalimumab (AbbVie) saw cheaper biosimilar products being chosen over their own. So, to stay competitive they are now also joining the price war by offering price reductions on their product of the previous list price.
All of this can only be a good thing for the patient. Cost is undoubtedly a big factor in whether or not a patient receives a biologic treatment for their IBD. With costs reducing, we may now see more access to this type of treatment for patients.
In the UK biologics are currently often reserved for the hardest to treat patients.
The traditional method of treating IBD sees biologics and biosimilars placed at the top of a step-up treatment pathway (see image below). If a patient is placed on a medication at the bottom of the pathway and their IBD doesn’t improve on it then they are often moved onto the medicine in the next tier, and so on. This is referred to as step-up treatment. Biologics are at the top of this, the last class of medication currently available to patients. One of the main reasons why it is placed at the top could be down to the cost of biologics.
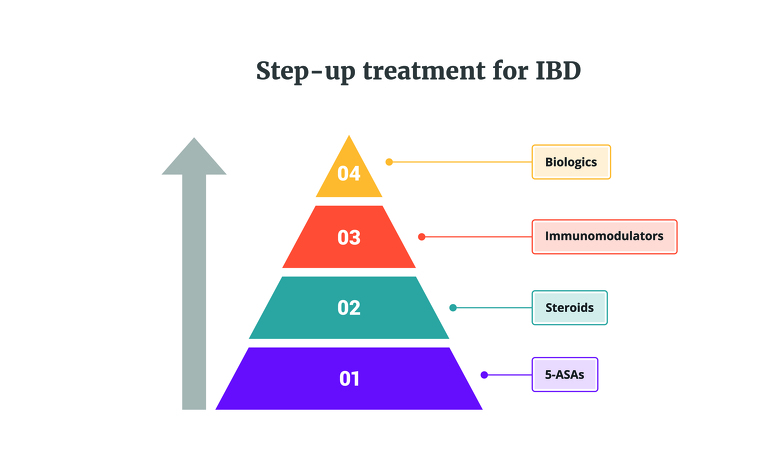
One study which followed a group of 1289 IBD patients over five years found that in year five 73% of the associated costs in Crohn’s disease were related to biologics and 48% in ulcerative colitis7. This cost had steadily increased over the years, suggesting the application of a step-up approach in treatment.
The National Institute for Clinical Excellence (NICE) in the UK reports that a 26 week cycle of a anti-TNF alpha medicine (such as infliximab) for ulcerative colitis costs approximately £4,900-£5,500 for an adult, while other conventional treatments cost £50-8508. When you look at the costs involved, it’s easy to see why it can be difficult for prescribers and patients to have access to biologics.
So does the introduction of infliximab and adalimumab biosimilars, and the associated decrease in costs, mean more patients are going to have earlier access to these medicines? Maybe.
As the cost gap between biologics and other IBD treatments reduces we may see more and more IBD teams being able to apply a ‘top-down’ pathway (starting with biologics first) rather than the current ‘step-up’ approach for the patients they consider to have moderate to severe IBD.
But, identifying exactly who the candidates for top-down treatment are is hard. Currently it’s a judgement call made by doctors based on their clinical experience, patient information and test results, to identify who may benefit from this early treatment with biologics.
It also wouldn’t necessarily be appropriate for all IBD patients to receive biologics as their first treatment. Biologics do come with some serious side effects - a concern both clinical teams and patients hold. Prescribing biologics in the first instance could also lead to ‘over-treatment’ of those patients who would do well on medications at the bottom of the IBD treatment pathway which may come with a less serious side effect profile.
Researchers and IBD teams continue to search for the answer to this problem. Recently prognostic tests have become available to predict future events in Crohn’s disease and ulcerative colitis, including whether a patient is likely to have relapsing disease (frequent flares) indicating more severe disease. However, they are not yet widely used by IBD teams as they aren’t reimbursed by the healthcare providers (meaning you have to pay for them yourself).
Tests such as these could herald the start of more precision medicine in IBD, where we are able to personalise treatments to the individual, rather than giving the same treatment to all. Research and technologies are certainly moving in this way, and the reduction in cost of biologics could be an important piece of the puzzle to get us there.